Client
Our Customer is a world leader in the production of injectable medicines and clinical nutrition, for both hospital and home therapies, surgical medical principals and transfusion products, with a focus on critical patients or patients affected by chronic diseases. The factory in which we operated is the customer’s factory where blood filters and conventional transfusion systems are produced.
Location: Mirandola (MO) – Italy
Client: World leader in the production of medical devices for blood products
Design: 2018
Completion: 2019
Designer: Palladio Consulting Srl
General contractor: confidential
Gross project site area: 600 m2
Project cost: confidential
Work carried out
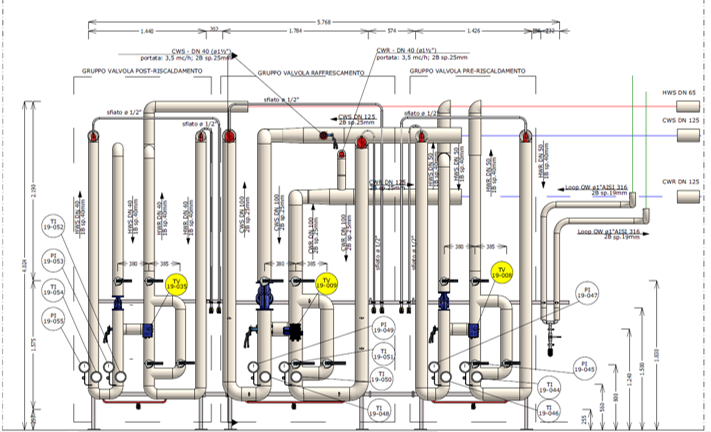
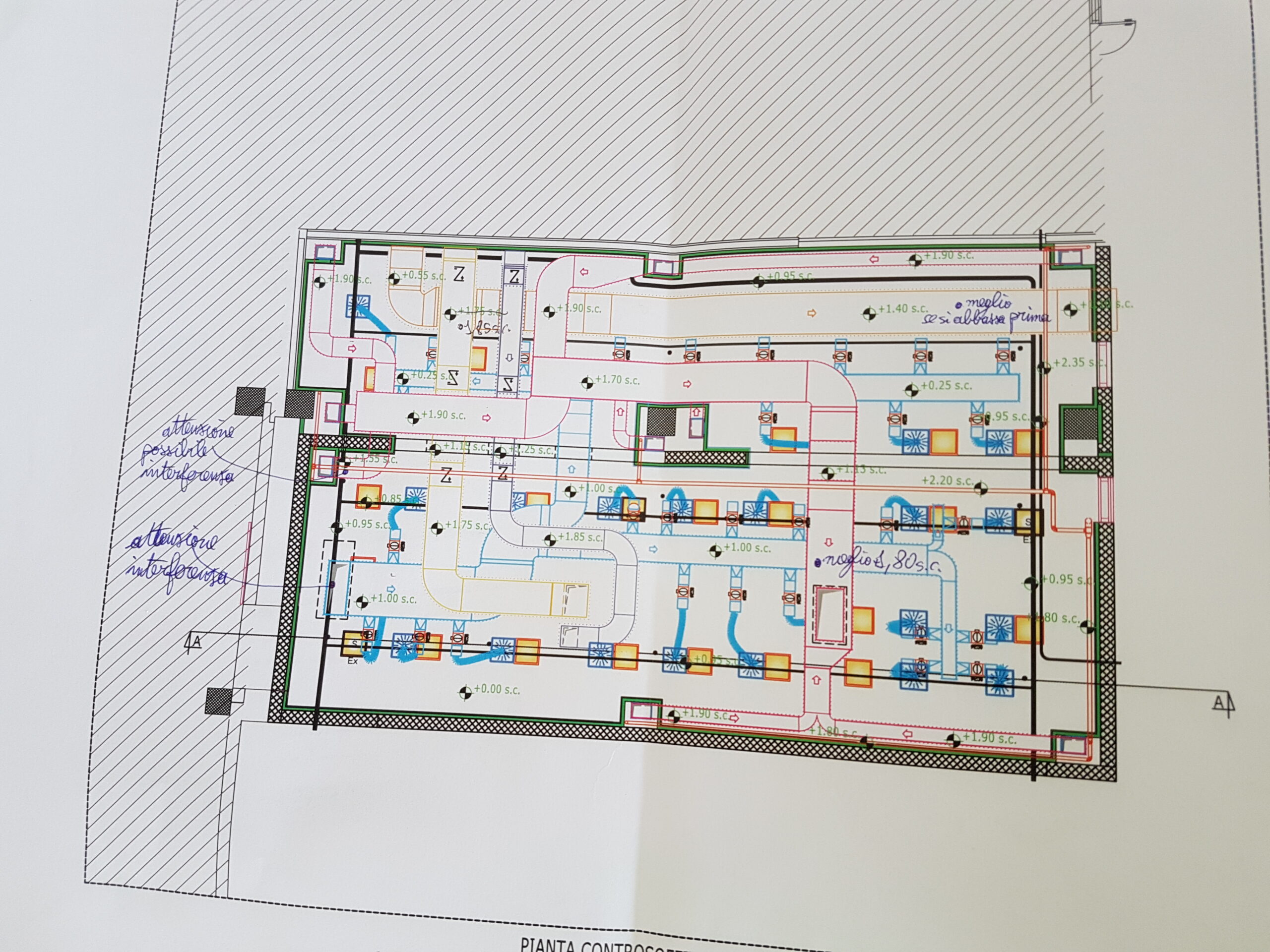
Our Customer has relied on us for engineering support of design, procurement assistance, job management, safety and qualification, with the EPCMV (Engineering, Procurement & Construction Management) formula for the construction of a new factory building attached to the existing in which a new ISO7 classified department attached to the existing production, an independent technology compartment and interconnecting utilities to the existing plant have been inserted. HWS-HWR/CWS-CWR), and clean utilities (PW, CA, LPS).
Palladio Consulting Srl took over the management and responsibility of the work, carrying out on-site inspections and technical meetings and coordinating structural and plant activities with contractors. Palladio designed and coordinated the construction of the civil works of foundation and elevation, in cement conglomerate and structural carpentry for light roofing for the plant located in Seismic Zone 3.